Longitudinal outcomes of cochlear implantation and bimodal hearing in a large group of adults: A multicenter clinical study
D. Kelsall, J. Lupo, A. Biever
Rocky Mountain Ear Center
Purpose
To measure preimplant speech recognition scores in 100 adults who showed poor hearing abilities with well fit hearing aids in unilateral hearing aid (HA) and bilateral hearing aid (2HA) listening conditions and postimplant speech understanding at 3-, 6- and 12-months for unilateral cochlear implant (CI) and bimodal (CI+HA) conditions.
Key messages
On average, by 3-months postimplant participants exhibited significant increases in speech recognition in the implanted ear compared to their preimplant performance with a hearing aid. Postimplant, group mean performance progressed significantly on all measures up to 12- months, with the largest and clinically notable advancements in the 3 – 6-month postimplant period.
At 6- and 12-months postimplant, bimodal hearing with the Cochlear™ Nucleus® CI532 implant, Nucleus® 7 Sound Processor and a ReSoundGN hearing aid provided significantly better group mean speech understanding compared to preimplant bilateral hearing aid performance. Counseling should incorporate the potential benefits of an implant in combination with a hearing aid for everyday listening.
Methods
- Prospective, non-randomized, multicenter controlled trial at 13 clinical sites
- 100 experienced hearing aid users > 18 years old with postlinguistic onset of bilateral, moderate sloping to profound sensorineural hearing loss
- Aided CNC word score < 40% in the ear to be implanted and < 50% in the contralateral ear
For all participants, hearing aids were fit to target and baseline speech perception measures completed in unilateral and bilateral listening conditions. All 100 enrolled participants received a Nucleus CI532 implant and a Nucleus 7 Sound Processor. Postimplant, 98/100 participants received a ReSoundGN HA for the contralateral ear. Two individuals had no aidable hearing in the contralateral ear. Daily bimodal hearing (CI+HA) was required for 6-months, then bimodal hearing was optional. At 12-months, participants were tested in the bimodal condition if they brought their HA.
Pre- and postimplant speech recognition in sound field was assessed in unilateral (HA, CI) and bilateral (2HA) /bimodal (CI+HA) conditions for CNC words in quiet at 60 dBA and AzBio sentences at 65 dBA in background noise at +10 and +5 dB SNR. Pre- and postimplant unilateral testing was conducted with the contralateral ear plugged. Bilateral evaluations occurred with 2HAs preimplant and CI+HA at 6-months (compulsory) and 12-months (optional). At the 6-month primary endpoint, CNC word scores for the implanted ear were compared to HA preimplant. All results represent matched pair data.
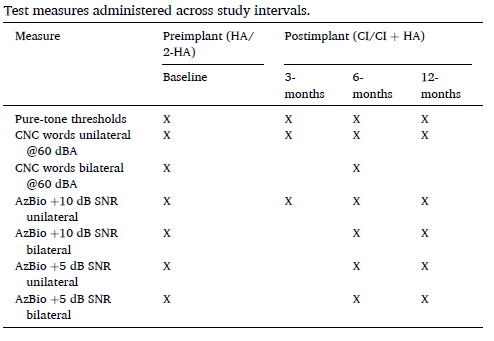
Table 1: Test measures administered across study intervals. © 2020 Published by Elsevier Inc.
Results
Ninety-six out of 100 subjects completed the 6-month endpoint visit. One subject withdrew due to an unrelated illness and three subjects underwent revision surgery with the Nucleus CI512 implant. In this study, subjects were seniors (average of 67 years old) and had a moderate-to-profound hearing loss in the implanted ear and a severe hearing loss in the contralateral ear for approximately 8 years in each ear. On average, 20 years of HA experience was reported for the implanted ear and 19 years for the contralateral ear.
When looking at the implanted ear (Figures 1 & 2), by 6 months after activation the average pre-operative CNC word score of 14.6% more than quadrupled to 60.9% in the implanted ear (improvement of 46.3 percentage points) and AzBio sentence scores at +10 SNR nearly tripled from 14.8% to 42.7% (improvement of 27.9 percentage points). Compared to preoperative scores, 93% and 73% of subjects performed significantly better on CNC words in quiet and AzBio sentences at +10 dB SNR respectively. The remainder showed equivalent scores on each test.
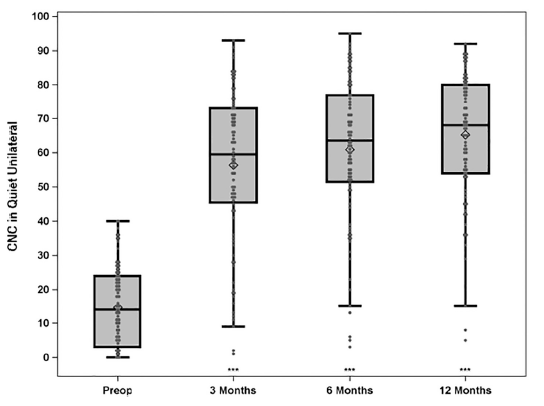
Figure 1: Group mean CNC percent correct word scores in quiet for implanted ear (preimplant HA and postimplant CI) for N = 96 at preimplant, postimplant 3- and 6-months and N = 91 at 12-months. Boxplots show the distribution of speech recognition scores with median, quartiles, minimum and maximum values. The mean is indicated with a diamond. Asterisks indicate a significant difference from preimplant at each post implant interval, ***p < 0.001. © 2020 Published by Elsevier Inc.
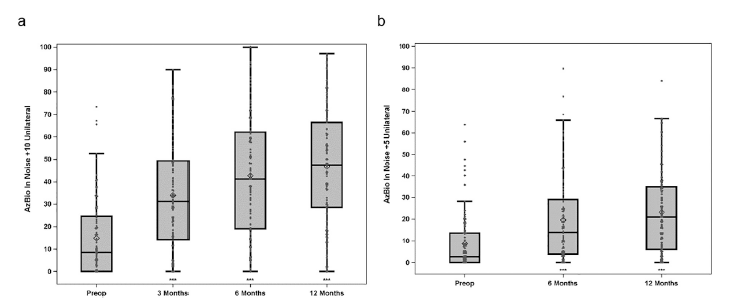
Figure 2 a, b: Group mean percent correct scores for AzBio sentences in noise at +10 (left) and +5 dB (right) SNR for implanted ear (preimplant HA and postimplant CI). Results are for N = 96 at preimplant and 3-months, N = 94 at 6-months and N = 91 at 12-months. Boxplots show the distribution of speech recognition scores with median, quartiles, minimum and maximum values. The mean is indicated with a diamond. Asterisks indicate a significant difference from preimplant at each post implant interval, ***p < 0.001. © 2020 Published by Elsevier Inc.
Using the bimodal solution of the Nucleus 7 Sound Processor and ReSoundGN hearing aid together (N=94), CNC word scores increased from 28.8% with HAs to 69.2% (improvement of 40.5 percentage points) and the average AzBio sentences +10 SNR score of 31.8% rose to 55.9% (improvement of 24.1 percentage points) as shown in Figures 3 & 4. Compared to preoperative scores, 87% and 70% of subjects performed significantly better on CNC words in quiet and AzBio sentences at +10 dB SNR respectively. Equivalent scores were demonstrated by 13% of subjects on CNC words in quiet.
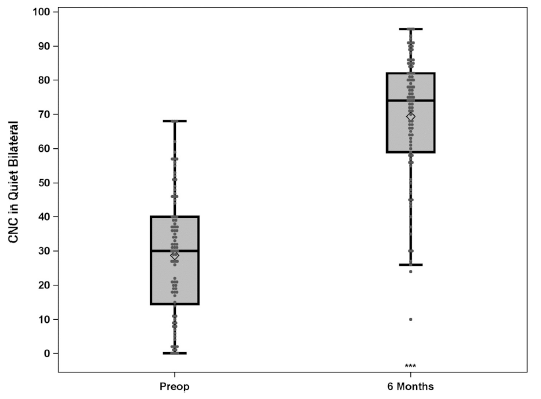
Figure 3: Group mean CNC percent correct word scores in quiet for preimplant bilateral hearing aids condition and bimodal condition (CI + HA) at 6-months postimplant. Results are for N = 94. Boxplots show the distribution of speech recognition scores with median, quartiles, minimum and maximum values. The mean is indicated with a diamond. Asterisks indicate a significant difference from preimplant to the 6-months postimplant interval, ***p < 0.001. © 2020 Published by Elsevier Inc.
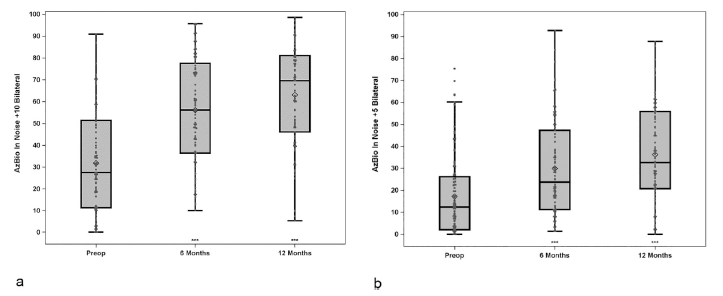
Figure 4 a, b: Group mean percent correct scores for AzBio sentences in noise at +10 (left) and +5 dB (right) SNR for bilateral hearing aids condition at preimplant, and bimodal condition (CI + HA) at postimplant. Results are from scores for N = 96 at preimplant, N = 94 at 6-months and N = 83 at 12-months. Boxplots show the distribution of speech recognition scores with median, quartiles, minimum and maximum values. The mean is indicated with a diamond. Asterisks indicate a significant difference from preimplant at each postimplant interval, ***p < 0.001. © 2020 Published by Elsevier Inc.
Conclusions
On average, participants experienced significant increases in speech understanding for words in quiet and sentences in noise while listening with the cochlear implant compared to their HA, and ongoing significant progress remained up to one year postimplant. Participants made most improvements in the first 6 months. Generally, compared to bilateral hearing aids, listening with the Nucleus 7 Sound Processor along with a ReSoundGN hearing aid provided significantly greater speech recognition at 6- and 12- months, especially in more difficult listening in noise conditions. Counseling should incorporate possible benefits of a cochlear implant along with a hearing aid for regular listening. Individuals who obtain limited speech recognition from well fit hearing aids should be referred for a cochlear implant candidacy evaluation.
To read more Science Spotlights visit Cochlear ProNews.
Citation: Kelsall D, Lupo J, Biever A. Longitudinal outcomes of cochlear implantation and bimodal hearing in a large group of adults: A multicenter clinical study. Am J Otolaryngol. 2021 Jan-Feb; 42(1):102773. doi: 10.1016/j.amjoto.2020.102773 Epub 2020 Oct 22. PMID: 33161258
This article is intended to serve as a resource for clinicians to help keep up to date with current clinical literature and is intended for professionals only. Clinical literature is based on research, which may include the experimental use of new or currently available products and technologies. Therefore, literature presented on this blog may represent use of Cochlear products that does not align with the intended use or indications approved by regulatory bodies, also known as off-label use. Cochlear does not condone any off-label use of its products, and it is not Cochlear’s intent to promote off-label use by providing this blog as a resource to clinicians.
This article summary is for education and information sharing amongst professionals and partners. Any representations made in relation to medical devices or therapeutic goods are not advertisements. The representations and views expressed in this summary are those of the authors and do not reflect the views of Cochlear. Cochlear does not endorse any particular treatment or research protocol nor off-label use.
This content is meant for professional use. If you are a consumer, please seek advice from your health professional about treatments for hearing loss. Outcomes may vary, and your health professional will advise you about the factors which could affect your outcome. Always read the instructions for use. Not all products are available in all countries. Please contact your local Cochlear representative for product information. Views expressed are those of the individual. Consult your health professional to determine if you are a candidate for Cochlear technology.