By Dr. Brian Kaplan, Senior Vice President of Clinical Strategy & Innovation at Cochlear
Unilateral Cochlear Implants for Bilateral Severe, Profound, or Moderate Sloping to Profound Sensorineural Hearing Loss: A Systematic Review and Consensus
Authors
An international group of clinical experts in the fields of otology, audiology and hearing science, all with extensive clinical and scientific experience of cochlear implantation, were brought together to form a Delphi panel. The aim of the group was to use a modified Delphi method to develop a series of consensus statements regarding the use of unilateral CIs for treating bilateral severe, profound or moderate sloping to profound sensorineural hearing loss (SNHL).
The aim of this paper is to describe the findings of the Delphi consensus study on cochlear implant use in adults and to present the resulting consensus statements agreed by the Delphi panel.
DELPHI GROUP: Craig A Buchman MD, René H Gifford PhD, David Haynes MD, Thomas Lenarz MD, Gerard O’Donoghue FRCS, Oliver Adunka MD, Allison Biever AuD, Robert Briggs FRACS,8 Matthew L Carlson MD, Pu Dai, MD, Colin Driscoll MD, Howard W Francis MD, Bruce Gantz MD, Richard K Gurgel MD, Marlan Hansen MD, Meredith Holcomb AuD, Eva Karltorp MD, Milind Kirtane MS ENT, Jannine Larky AuD, Emmanuel Mylanus MD, J Thomas Roland Jr MD, Shakeel R Saeed MD, Henryk Skarzynski MD, Piotr H Skarzynski MD, Mark Syms MD, Holly Teagle AuD, Paul Van de Heyning MD, Christophe Vincent MD, Hao Wu MD, Tatsuya Yamasoba MD, Terry Zwolan PhD.
What was the goal of the consensus paper?
Consensus statements were developed regarding the use of unilateral cochlear implants in adults with severe, profound or moderate sloping to profound bilateral sensorineural hearing loss.
Why is this important?
The consensus statements provide recommendations on the use of unilateral cochlear implants in adults with bilateral severe, profound or moderate sloping to profound sensorineural hearing loss. They could inform the development of clinical practice guidelines, which could increase access to cochlear implants worldwide and improve hearing and quality of life in eligible adults.
How did they do it?
A Delphi consensus panel of 30 international specialists voted on statements about cochlear implant use, informed by a systematic review of the literature and clinical expertise. This resulted in 20 evidence-based statements that are in line with real-world clinical experience.
Systematic Review (SR) of the Literature
A Systematic Review was performed to identify studies relevant to at least one of the following six key areas: i) level of awareness of CIs; ii) best practice clinical pathway from diagnosis to surgery; iii) best practice guidelines for surgery; iv) best practice guidelines for rehabilitation; v) factors that affect CI performance and outcomes; and vi) cost implications of CIs.
The literature searches were conducted on July 18, 2018, in the following electronic databases. i) MEDLINE In-Process & Other Non-Indexed Citations and OVID MEDLINE, 1946–present; ii) Embase, 1974–present; and iii) Cochrane Library, comprising Cochrane Database of Systematic Reviews, Database of Abstracts of Reviews of Effects, Cochrane Central Register of Controlled Trials, National Health Service Economic Evaluation Database, the Health Technology Assessment database, and the American College of Physicians Journal Club.
Eligibility Criteria
The title and abstract of the identified publications were screened manually against prespecified eligibility criteria. The searches were limited to human studies published in English language and conducted in Australia, Canada, China, Europe, India, Japan, the UK, and the USA. SRs of observational studies, prospective and retrospective studies, and cross-sectional and longitudinal studies were included. Full-text versions of all publications meeting the eligibility criteria at initial screening were reviewed to confirm eligibility. Key exclusions were: studies with sample sizes smaller than 20; case studies, case series and narrative reviews; studies published before 2005 (so that only studies on new-generation technology were included); pediatric populations; studies of hearing preservation; bilateral CIs; electroacoustic stimulation or hybrid hearing; and single-sided deafness with tinnitus suppression.
Data Extraction and Statement Development
Data relevant to the six key areas of interest were extracted manually from the included studies, and consensus statements were drafted based on the findings.
Quality Assessment of the Evidence
Quality assessment was conducted on all included studies at the full-text review stage, using a modification of the method outlined in Eubank et al. 2016.22 This method has previously been used in a Delphi consensus study 22 and includes assessment criteria for a wide range of study types.
The literature was rated on the basis of study design, assigning the study with a numerical score from 1 to 5, with 1 being the highest-quality evidence and 5 being the lowest-quality evidence.
The Eubank method was adapted to include survey-based studies, which were ranked as level 5 because they generate data based on expert opinion. The method was also modified to include economic-based studies and to differentiate between retrospective prognostic studies (level 2) and retrospective therapeutic studies (level 3), as described by Wright et al. 2003.23
The Quality Assessment rating for the evidence supporting each statement was made available to the Delphi panel at each voting stage.
Results
In total, 6,492 papers were identified in the searches of the electronic literature databases. After removal of duplicates, 74 articles fulfilled all the inclusion criteria and were used to create these statements. Some of these papers were relevant to more than one category. Twenty statements on the use of unilateral cochlear implants in adults with sensorineural hearing loss were developed, relating to:
Consensus Statement Categories
- Level of awareness of CIs
- Best practice clinical pathway for diagnosis
- Best practice guidelines for surgery
- Clinical effectiveness of CIs
- Factors associated with post-implantation outcomes
- The relationship between hearing loss and depression, cognition and dementia
- Cost implications of CIs
Consensus Statements
Category 1 – Level of awareness of CIs
1. Awareness of cochlear implants among primary and hearing healthcare providers is inadequate, leading to under-identification of eligible candidates. Clearer referral and candidacy pathways would help increase access to cochlear implants.
Category 2- Best practice clinical pathway for diagnosis
2. Detection of hearing loss in adults is important; pure tone audiometry screening methods are considered the most effective. The addition of a questionnaire or interview to the screening can improve the detection of sensorineural hearing loss.
3. Preferred aided speech recognition tests for cochlear implant candidacy in adults include monosyllabic word tests and sentence tests, conducted in quiet and noise. Further standardization of speech recognition tests is needed to facilitate comparison of outcomes across studies and countries.
4. Age alone should not be a limiting factor to cochlear implant candidacy, as positive speech recognition and quality of life outcomes are experienced by older adults as well as younger adults.
Category 3 – Best practice guidelines for surgery
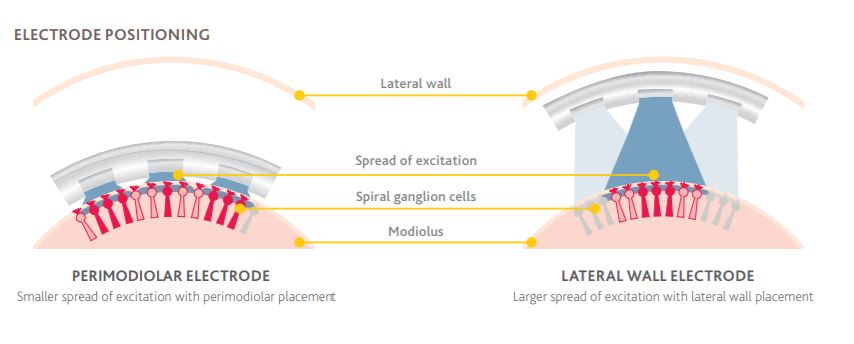
5. Both curved (perimodiolar) and straight electrodes are clinically effective for cochlear implantation, with a low rate of complications.
6. When possible, hearing preservation surgery can be beneficial in individuals with substantial residual hearing.
Category 4 – Clinical effectiveness of CIs
7. Cochlear implants significantly improve speech recognition in both quiet and moderate noise in adults with bilateral severe, profound, or moderate sloping to profound sensorineural hearing loss. These gains in speech recognition are likely to remain stable over time.
8. Both word and sentence recognition tests should be used to evaluate speech recognition performance following cochlear implantation.
9. Cochlear implants significantly improve overall and hearing-specific quality of life in adults with bilateral severe, profound or moderate sloping to profound sensorineural hearing loss.
10. Adults who are eligible for cochlear implants should receive the implant as soon as possible to maximize post-implantation speech recognition.
Category 5 – Factors associated with post-implantation outcomes
11. Long durations of unaided hearing loss do not rule out potential benefit of cochlear implants: individuals who receive an implant in an ear that was previously unaided for more than 15 years have been shown to experience improvements in speech recognition.
12. Adults who have undergone cochlear implantation should receive programming sessions, as needed, to optimize outcomes.
13. Where appropriate, individuals should use hearing aids with their cochlear implant in order to achieve bilateral benefits and the best possible speech recognition and quality of life outcomes.
14. Many factors impact cochlear implant outcomes. Further research is needed to understand the magnitude of the effects.
Category 6 – The relationship between hearing loss and depression, cognition, and dementia
15. Adults with hearing loss can be substantially affected by social isolation, loneliness and depression. Evidence suggests that treatment with cochlear implants can lead to improvement in these aspects of well-being and mental health. Longitudinal studies are needed to obtain further knowledge in this area.
16. There is an association between age-related hearing loss and cognitive/memory impairment.
17. Further research is required to confirm the nature of cognitive impairment in individuals with hearing loss, and its potential reversibility with treatment.1
18. The use of cochlear implants may improve cognition in older adults with bilateral severe to profound sensorineural hearing loss.
19. Hearing loss is not a symptom of dementia. However, treatment of hearing loss may reduce the risk of dementia.
Category 7 – Cost implications of Cis
20. Unilateral cochlear implantation in adults is cost-effective when compared with no implant or no intervention at all and is associated with increased employment and income.
Discussion
These consensus statements represent the first step toward the development of international guidelines on best practice for cochlear implants in adults with sensorineural hearing loss. Further research to develop consensus statements for unilateral cochlear implants in children, bilateral cochlear implant, combined electric-acoustic stimulation, unilateral implantation for single-sided deafness and asymmetrical hearing loss in children and adults will be beneficial for optimizing hearing and quality of life for these patients.
References
Delphi Consensus Group. April 2020. Unilateral Cochlear Implants for Bilateral Severe, Profound, or Moderate Sloping to Profound Sensorineural Hearing Loss: A Systematic Review and Consensus. JAMA OTOLARYNGOLOGY – HEAD AND NECK SURGERY.
1Washington University School of Medicine, St. Louis, MO, USA; 2Vanderbilt University Medical Center, Nashville, TN, USA; 3Hannover Medical School, Hannover, Germany; 4University of Nottingham, Nottingham, UK; 5Nottingham University Hospitals NHS Trust, Nottingham, UK; 6Ohio State University, Columbus, OH, USA; 7Rocky Mountain Ear Center, Englewood, CO, USA; 8The University of Melbourne, Melbourne, VIC, Australia; 9Royal Victorian Eye and Ear Hospital, Melbourne, VIC, Australia; 10Royal Melbourne Hospital, Melbourne, VIC, Australia; 11Mayo Clinic School of Medicine, Rochester, MN, USA; 12General Hospital of People’s Liberation Army, Beijing, China; 13Duke University School of Medicine, Durham, NC, USA; 14University of Iowa, Iowa City, IA, USA; 15University of Utah Hospital, Salt Lake City, UT, USA; 16Medical University of South Carolina, Charleston, SC, USA; 17University of Miami, Miami, FL, USA; 18Karolinska University Hospital, Stockholm, Sweden; 19Seth Gordhandas Sunderdas Medical College and King Edward Memorial Hospital, Mumbai, India; 20Stanford University School of Medicine, Stanford, CA, USA; 21Radboud University Medical Centre, Nijmegen, Netherlands; 22New York University School of Medicine, New York, NY, USA; 23University College London Ear Institute, London, UK; 24Institute of Physiology and Pathology of Hearing, World Hearing Center, Kajetany, Nadarzyn, Poland; 25Medical University of Warsaw, Warsaw, Poland; 26Institute of Sensory Organs, Kajetany, Nadarzyn, Poland; 27Arizona Hearing Center, Phoenix, AZ, USA; 28University of Auckland, Auckland, New Zealand; 29Antwerp University Hospital, University of Antwerp, Edegem, Belgium; 30Centre Hospitalier Universitaire de Lille, Lille, France; 31Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China; 32University of Tokyo Hospital, Tokyo, Japan; 33University of Michigan, Ann Arbor, MI, USA.
Funding/Support
Funding was provided by Advanced Bionics (Valencia, CA, USA), Cochlear Ltd (Sydney, NSW, Australia), MED-EL (Innsbruck, Austria), and Oticon Medical (Smorum, Denmark).
Role of Funder/Sponsor
Advanced Bionics (Valencia, CA, USA), Cochlear Ltd (Sydney, NSW, Australia), MED-EL (Innsbruck, Austria), and Oticon Medical (Smorum, Denmark) provided funding for assistance with the preparation of the manuscript and were informed of the decision to submit the manuscript for publication; they had no input to the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. There was no remuneration of the Delphi participants including the Chair and Committee members.
Cochlear Nucleus cochlear implants are intended for use in individuals 18 years of age or older who have bilateral, pre, peri or postlinguistic sensorineural hearing impairment and obtain limited benefit from appropriate binaural hearing aids. These individuals typically have moderate to profound hearing loss in the low frequencies and profound (≥90 dB HL) hearing loss in the mid to high speech frequencies.